Major elective surgery in children, and surgery in remote and rural locations
This page is under construction, converting the originally formatted pdf from the WFSA site with wiki embellishments.
Originally from Update in Anaesthesia | www.wfsahq.org/resources/update-in-anaesthesia
Mark Newton
Vanderbilt University, Department of Anesthesiology and Pediatrics (USA)
AIC Kijabe Hospital, Department of Anesthesiology
Correspondence Email: mark.w.newton@Vanderbilt.edu
| Summary |
|---|
| Children presenting for elective paediatric surgery in sub-Saharan Africa may have a high morbidity and mortality. Understanding the basic anatomy, physiology and pharmacology together with appropriate equipment for the paediatric patient will improve the anaesthesia mortality statistics. The development of paediatric surgical centres in both the rural and urban settings will allow for greater experience to be obtained in paediatric anaesthesia, which will improve care. The most valuable asset for these paediatric centres is to have well-trained physicians and nurses who can provide high quality care for children with the advanced surgical pathology encountered, taking account of the lack of infrastructure and the limited supplies that are a common problem. A successful perioperative course can be expected even for children requiring surgical intervention in austere environments. |
Introduction
Safe, effective care of children presenting for surgery is challenging, even more so when the child presents with advanced pathology, the systems to support safe anaesthesia care are not well-developed, and the need for surgery is large. In many low-income countries, more than 50% of the population is less than 15 years old, and more than 85% of children are predicted to need some form of surgical intervention by the age of 15. The overall morbidity and mortality in this group of patients is alarmingly high when compared to similar patients in high-income countries, and the mortality would be expected to be even higher in the emergency cases.
Elective paediatric cases in children older than 6 months old are performed at many hospital levels in the rural and the urban setting. Common elective procedures in rural hospitals include:
- Inguinal and umbilical hernia
- Circumcision
- Incision and drainage of soft tissue and orthopaedic infections.
Larger more challenging cases often undertaken in urban centres include:
- Hypospadias repair
- Excision of abdominal mass (e.g. mesenteric cyst, Wilm’s Tumor, ovarian mass)
- Head and neck surgery.
These patients are likely to be challenging even in a large national referral centre and good understanding and preparation is required.
This article will give a brief overview of the general principles of anaesthesia in children, and describe some clinical cases with practical solutions to equipment requirements and clinical goals, which we hope will be useful to anaesthesia providers working in any setting.
General Principles
Cardiac
Infants and young children have a relatively fixed cardiac output compared to adults due to immature myocardial function. The resting heart rate is high and there is limited ability to increase the stroke volume in response to a fluid challenge. Babies become bradycardic (heart rate slows) in response to hypoxia, but children older than 6 months of age have developed a balance between the sympathetic and parasympathetic nervous systems, and have the more usual tachycardia in response to hypoxia. The fall in the heart rate due to hypoxia in babies causes a fall in blood pressure; your immediate response should be to control the ventilation with 100% oxygen, or whatever level of oxygen is available in your setting, rather than attend to the blood pressure – i.e. you should not grab for the atropine but rather open the airway and/or assist ventilation, as hypoxia will be the source of the bradycardia in the vast majority of the situations. Your response to hypoxia must be rapid as babies have twice the oxygen consumption of adults, and quickly become desaturated. Always prepare your age appropriate airway equipment and full range of drugs prior to the start of a case, and make sure you have an assistant.
Children presenting for elective surgery, particularly those with congenital conditions associated with midline defects such as hypospadias, cleft lip and cleft palate, may also have associated congenital heart disease (CHD). If a murmur is present, or the child has a history suggestive of cardiac disease (failure to thrive, frequent chest infections and/or cyanosis), then a further work-up may be indicated prior to surgical intervention. Most children will present to a general hospital without an adult cardiologist, let alone a paediatric cardiologist. Work-up for such a child should include a good history, including questions about feeding, weight gain, chest infections, ‘funny turns’ and exercise tolerance; examination to listen for murmurs or cyanosis and clubbing; chest Xray (to exclude cardiomegaly or pulmonary edema); and room air oxygen saturation measured with a pulse oximeter to exclude cyanotic heart disease or a chest infection in a child with cardiac failure. If the child is well and asymptomatic, not cyanosed, the murmur is not loud, not diastolic, there is no thrill and the chest Xray is normal, it should be safe to proceed as the murmur is likely to be benign. These cases will not require antibiotic prophylaxis unless the surgeons request an antibiotic for their surgical procedure.
Acquired rheumatic valvular heart disease is always a possible finding in a low resource setting, as the prevalence of this disease is higher and is increasing. If the patient is asymptomatic with good exercise tolerance and oxygen saturation, an elective surgical case can be performed safely.
Respiratory
Anaesthetic induction and intubation in babies requires special care because the oxygen saturation will decrease much faster than in an adult due to the higher oxygen consumption, high minute ventilation and reduced functional residual capacity (FRC) per kg body weight compared to adults.
The narrowest part of the airway in the child is the cricoid cartilage, not the vocal cords as in the adult. The glottis is more anterior and the epiglottis is less rigid and tends to fall back to occlude the glottic opening during intubation. The large occiput in infants tends to cause neck flexion, the tongue is relatively large, and it is easy to press on the floor of the mouth to push the tongue up, which means the airway is easily obstructed during facemask anaesthesia. The airway cartilages are soft and easily compressed; if cricoid pressure is used, the pressure should be gentle otherwise the trachea can collapse and you may not be able to ventilate due to obstruction of the trachea itself. Lateral displacement is also a frequent factor when an assistant is applying cricoid pressure with too much enthusiasm. If this is the case, ask your assistant to release the pressure to improve your view of the larynx. The combination of these anatomical differences and the limited oxygen reserve, relatively high oxygen requirement, and poor tolerance of hypoxia means that intubation can be more difficult in a neonate compared to an adult, but with skill and experience, safe intubation becomes routine.
The following airway equipment must always be prepared and checked prior to surgery. This preparation is a necessary, essential and professional aspect of the anaesthetic plan:
- Stethoscope
- Pulse oximeter
- Functioning laryngoscope with blades
- Endotracheal tube and stylet
- Oropharyngeal airways
- Mask.
It is essential to have the appropriate sized paediatric airway equipment available, including laryngoscope blades (Miller 0, 1, 2 blades), endotracheal tubes, facemasks and oral airways. A stylet can be helpful but, if not available, can be made with a flexible metal tube that has been blunted on both ends to prevent tracheal damage or endotracheal tube perforation. A precordial stethoscope and a pulse oximeter are essential monitoring equipment for all cases.
It is essential to have the appropriate sized paediatric airway equipment available, including laryngoscope blades (Miller 0, 1, 2 blades), endotracheal tubes, facemasks and oral airways. A stylet can be helpful but, if not available, can be made with a flexible metal tube that has been blunted on both ends to prevent tracheal damage or endotracheal tube perforation. A precordial stethoscope and a pulse oximeter are essential monitoring equipment for all cases.
The technical skill of intubation should be practised in adults or older children before attempting to perform even an elective intubation in a small child; many paediatric airway disasters could have been avoided with better training, also with assistance on induction. The anaesthetist should always ask the surgeon or a nursing colleague to help with induction and intubation as respiratory disasters can happen very quickly in children.
Renal
Glomerular filtration rate (GFR) reaches adult levels by one year of age, and most children presenting for elective surgery have normal renal function. Routine questions such as “Any wet diapers/nappies, and when?” will be useful to assess hydration and renal function healthy children. Routine electrolytes and creatinine are not necessary except for in renal surgery where it is useful to record baseline values. A urinary catheter is rarely required, except for hypospadias repair or Wilm’s tumours. The smaller sizes of urinary catheter are often not available, and it is much better to avoid damage to the urethra rather than to insert an inappropriately large catheter. The bladder can be emptied by the surgeon pressing gently on the lower abdomen during the case (if the area is surgically prepped), and urine collected in a diaper. Urine output can be estimated from the difference in weight of the diaper pre-surgery and post-surgery; one mg increase in weight in the diaper is equivalent to one ml of urine. A scale capable of measuring small weights must be used. All doses of drugs should be calculated and drawn up accurately, especially renal toxic drugs such as gentamicin.
Fluid balance should be assessed carefully before surgery, particularly if the child has been starved for a long period of time; many children are starved for far too long preoperatively. Patients in an arid climate often have a chronically low intravascular volume; the additive effect of the pre-existing deficit and the fasting deficit may be revealed on induction of anaesthesia with inhalational agents such as halothane, which results in a dramatic fall in blood pressure. A bolus dose of 10- 20ml.kg-1 0.9% saline or Ringer’s provides adequate hydration (and rehydration) for most minor elective cases. Weigh swabs and assess fluid balance carefully for children undergoing major surgery with on-going fluid and blood losses. Most children presenting for elective general surgery do not require dextrose containing fluids as the stress response to surgery causes the blood sugar to increase; neonates or malnourished children should have their blood sugar checked to make sure they are not hypoglycaemic before surgery starts.
Make sure the child is well hydrated at the end of surgery; and, if possible, avoid IV fluids on the ward unless the child has undergone major surgery and is not able to drink. This is important even in the elective patient as it is difficult to monitor IV fluids on the wards as the patient:nurse ratio is often very high. Patients frequently do not get an intravenous line in good time, the IV fluids may not be available or may not be infused accurately.
Temperature Regulation
Temperature monitoring is not available in many countries, but low technology devices to detect hypothermia are available, and valuable, particularly in younger children. A simple thermometer can be used for axillary skin temperature measurement to help monitor trends. Children have a relatively large surface area and little fat for insulation, especially if malnourished. This can lead to dangerous hypothermia (low temperature) during surgery, which may result in clotting problems, hypoventilation and even cardiac arrhythmias. Basic heating pads and fluid warmers are helpful but need very close monitoring as they may also cause burns if not used properly. In particular, the heating pad should never be applied directly to the skin, and rarely placed on “high”.
Intraoperative hypothermia can be avoided in the following ways:
- Avoid excessive betadine to ‘prep’ the surgical field. If you are not watching the surgical technician’s use of betadine, the patient will be floating in cold fluid for the entire case. Pooling of betadine (or alcohol) against the child’s skin may also cause a chemical burn.
- If possible, warm the theatre (22-25°C, depending on the age of the child). An ambient heating unit is useful to warm the room and reduce early heat loss.
- Do not let the room get too cold. An open window producing a breeze for the surgical team may cool the patient down too much. If air conditioning is available, make sure the temperature is not turned down too low.
- Keep the patient covered with warm sheets whenever possible, including at the start of the case when IV lines are being inserted, and/or prior to cleaning and draping by the surgical team. It is very helpful if the early heat loss caused by vasodilation at the start of anaesthesia can be reduced.
Haematology
Anaemia with haemoglobin level (Hb) less than 8g.dl-1 is common in children presenting for elective surgery. Most will be nutritional, but you should consider other causes such as:
- Malaria
- Sickle cell disease
- Intestinal worms
- Drug-induced anaemia (rare)
It is feasible for children to have elective surgery when their haemoglobin is less than 8 g.dl-1, but they have reduced tissue oxygen delivery, and may need supplemental oxygen to maintain oxygen saturations, especially if they have upper abdominal surgery and impaired respiratory function due to pain. Minor procedures such as hernia repair can be undertaken safely with Hb as low as 6-7g.dl-1, but any major surgery needs a starting Hb above 8g.dl-1. If the child is anaemic and is presenting for elective surgery, and they live relatively close to the hospital, they should be treated with a course of iron supplements for 3 months before re-booking.
Acute malaria can produce unexpected complications and increased morbidity. All children presenting for elective surgery who have malaria should be treated and surgery postponed.
Sickle disease is associated with increased perioperative complications, usually due to sickle chest or painful crisis. Children with sickle disease presenting for elective surgery should not be allowed to become dehydrated, and should be transfused using fresh whole blood if the Hb is below 8g.dl-1. Ideally, freshly donated blood should be used for sickle cell disease patients, particularly if it is not possible to measure the sickle status of the donated blood. Typically a top up transfusion of 20ml.kg-1 will be sufficient, along with the normal sickle cell precautions. If the temperature is high after blood transfusion in a malaria endemic area, the child should have blood taken to test for malaria parasites and treatment for malaria considered.
Clinical Cases
Case 1
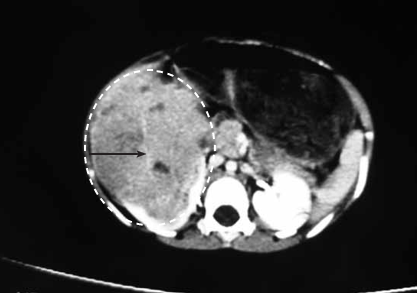
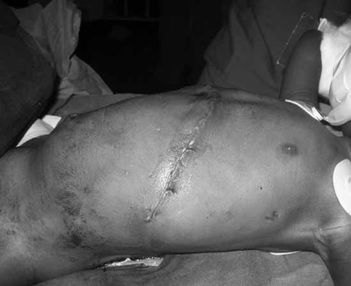
A three-year-old male child was referred to a tertiary referral hospital in East Africa with an 8 month history of enlarging abdominal mass. The child was previously healthy, travelled from a neighbouring country, had been examined by multiple medical care providers, and was very malnourished. His mother came with a CT scan of the mass and an exploratory laparotomy was scheduled by the surgical team. See Figure 1.
Preoperative Evaluation and Plan
Nephroblastoma (Wilms’ Tumor) is the most common abdominal tumour in children, with a reported incidence of 1:20,000 to 1:100,000. Typically tumours in sub-Saharan Africa are more advanced and in Nigeria, nephroblastoma is reported to be Stage III and IV in 72% of the children with an undocumented overall survival.[1] Advanced disease suggests suggests delayed presentation, poor surgical infrastructure or, possibly, more aggressive tumours compared to children in western research studies. Anaesthesia and surgery for advanced tumour cases can be very challenging.
A CT scan and a chest Xray are essential to plan surgery, to determine the mass effect of the tumour, location within the vascular system (e.g. identify involvement of the inferior vena cava or renal vein), presence of bilateral renal involvement (8- 12% of cases), and spread to the pulmonary parenchyma. Each of these would help determine if surgery should be altered or even cancelled due to extensive spread and unnecessary risks, although a surgical palliative mass debulking could still be considered. Many of these patients present in a state of malnutrition and their response to inhalation agents such as halothane may be more dramatic with more cardiovascular depression and drop in blood pressure than anticipated.
Each child needs an accurate weight documented in the chart for drug dosing, fluid requirements, and overall health assessment. Essential laboratory measurements include: haemoglobin, platelet count, creatinine (allows comparison of pre and postoperative renal function) and blood type and cross match, anticipating the potential for significant blood loss. A minimum of two adult units of type specific blood must be ready prior to skin incision. In addition, two family members with a matching blood type, or other appropriate donors, must be available in the theatre waiting area, with direct communication from the surgical team. You will need to have a minimum of two blood transfusion sets in theatre, in case one becomes obstructed with blood clots during the case. Additional labs are unnecessary and costly.
Postoperative care must be planned before surgery, including where the child will be cared for after surgery. The postop ward must allow for close observation with access to:
- Oxygen
- Suction
- Bed which has the capability to elevate the head (improving respiratory efforts)
- Focused nursing care and monitoring of vital signs.
This is often the key component to a successful hospital discharge from the surgical procedure and should never be overlooked in the preparatory phase of an anaesthesia plan. Many hospitals do not have an ICU, but good care is possible as a substitute for a formal ICU if the patient is positioned in the area closest to the nursing station with access to close monitoring. The vital signs and fluid input and output must be monitored (bladder catheter required), and a key person identified who will show an active response to the postoperative observations.
Induction and Maintenance of Anaesthesia
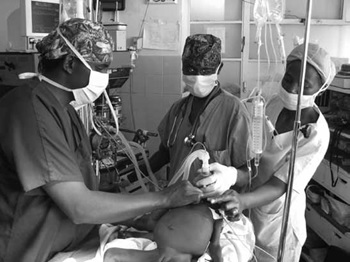
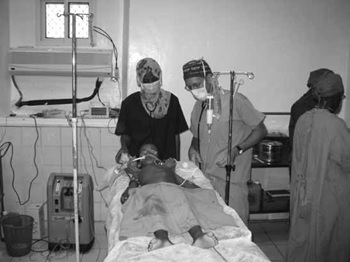
A large intra-abdominal tumour may predispose the patient to regurgitation of gastric contents on induction of anaesthesia. The diaphragm will be pushed up which will limit the lungs from fully expanding thus causing a faster oxygen desaturation. Both of these factors should prompt the anaesthesia care provider to ask for assistance during the induction phase of anaesthesia. (See Figure 2)
This patient should have an IV induction with careful application of cricoid pressure (NOT an inhalation induction). Remember, if you are having difficulty viewing the glottis, ask your assistant to reduce the cricoid pressure and/or change their compression direction to a more midline position. This patient could have any induction agent (thiopentone, propofol, or ketamine); and muscle relaxation (succinylcholine or rocuronium) to provide good conditions for rapid intubation.
I would not use any narcotics at this stage, but would wait until the airway has been secured. Children can have a more dramatic drop in oxygen saturation when they are apnoeic compared to adults, due to higher oxygen consumption, and in this case, the child will also have a reduced functional oxygen reserve, so will require efficient intubation. If the mass is very large, the head of the bed can be elevated slightly to reduce the effect of the mass compressing on the diaphragm, which may assist during the induction period. The lung volumes will be reduced due to elevation of the diaphragm, so check more than once that the endotracheal tube is not down too far and is in the proper position in the trachea. This patient will do best with a cuffed endotracheal tube, if available, due to increased intra-abdominal pressure during surgical tumour manipulation. If an uncuffed endotracheal tube is all that is available, place the appropriate size tube that only has a leak around the tube at higher pressures (between 20-30cmH20).
Higher inspiratory pressures than normal may be required due the mass effect of the tumour on the lungs, as would apply to any intra-abdominal pathology such as bowel obstruction that pushes up on the diaphragm. If the chest is not moving well, recheck the position of the endotracheal tube and adjust the inspiratory pressure; this should be undertaken as a priority rather than waiting for desaturation or carbon dioxide retention to occur.
Two large bore intravenous catheters should be inserted into the upper limbs for surgery. The cannulas are placed in the hands or arms because the tumour could involve the inferior vena cava (IVC), which will then need to be clamped for surgery. If the IV access is in the legs in this situation you would not be able to transfuse fluid to reach the central circulation and your lines would be useless.
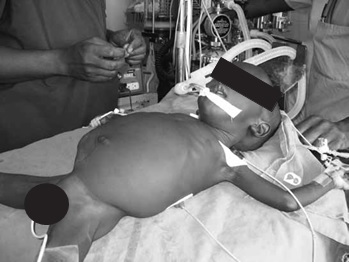
During the surgical exposure of the tumour, the surgical team could decrease venous return to the heart by compression of the IVC or by torsion on the liver, which would result in a sudden drop in the blood pressure without any signs of blood loss. You must watch the surgery closely so that you can anticipate blood loss and be aware of the manipulation of the tumour; you should alert the surgeons when the blood pressure drops. There will be times when you need to have the blood in the room and be ready to transfuse. If you are warming the blood in a bath of warm water, make sure that it is not too hot; if you cannot keep your hand in the water for more than 5 seconds then it is too hot and must not be used as you can cause haemolysis and massive infusion of potassium. Remember that 98% of the potassium in blood is intracellular; if the blood becomes haemolysed, the potassium will flood out of the cells and cause arrhythmias and even cardiac arrest when you transfuse the blood. With this child, the blood will need to be given in a 30-60 ml syringe, so that you can keep an accurate measurement of blood transfusion volume. Ideally, place a three-way stop cock in the infusion line, which will allow you to keep the syringe attached and to aspirate from the bag and infuse into the patient without a break in the blood line.
Children having major tumour excision need to have a urinary catheter inserted. An arterial line is not possible in many settings, so accurate non-invasive blood pressure monitoring needs to be done every two minutes, ideally using an automated cuff.
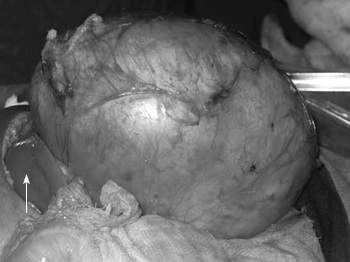
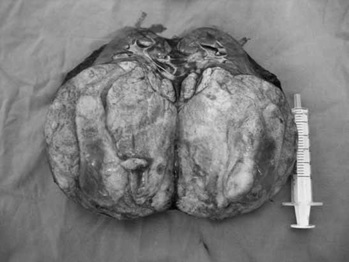
As one can see in the pathological specimen, these tumours will involve a large section of the kidney and one can see haematuria at times. In cases of bilateral tumour involvement, the surgeons may need to do renal sparing procedures (hemi-nephrectomy), which can be associated with very large blood loss and high risk for renal dysfunction postoperatively.
If the surgery is successful and the blood loss is minimal, the child can be extubated at the end of the procedure but needs to have good pain management. It is helpful if, in addition to opioids given during the procedure, the surgeon infiltrates the wound edges with a safe dose of local anaesthetic at the end of surgery (lignocaine 2% 3mg.kg-1 or bupivacaine 0.25% 2mg.kg-1). The surgery will be associated with significant postoperative pain, which should be managed by small doses of morphine or pethidine titrated to effect in the recovery room. If morphine is the drug available, a dose of 0.05mg.kg-1 should be given then an observation period before adding to reach a maximum does of 0.1mg.kg-1 every 4-6 hours depending upon the patients condition. Postoperatively, these patients need to be observed in a setting with a higher nurse to patient ratio, with a bed that can have the head elevated, oxygen in the room, and careful monitoring of fluid intake and output by the nursing team. If close observation is not possible, intramuscular opioids, at the appropriate dose, may be safer than intravenous narcotics in these settings. The appropriate dosing based upon accurate weight is critical when dealing with the paediatric surgical patient. The surgeons will usually request a nasogastric tube to be inserted as the child is likely to have a postoperative ileus after this large intra-abdominal tumour is removed.
Case 2
A 6-year-old female living in a very rural and resource poor area of Africa has had a one year history of abdominal swelling which has not responded to medical treatment. She is afebrile, with normal vital signs, no past medical or surgical history, and neither the family nor the medical facility has access to CT or MRI. She has travelled for two days to for a surgical consult by your outreach team as the area she lives in has minimal access to paediatric surgery. A portable ultrasound machine revealed a large intra-abdominal cystic mass and the surgeon would like to proceed to surgery. The hospital is without piped gases or oxygen tanks, no anaesthesia machines, and has one electrically powered oxygen concentrator that produces flow up to 6 litres.minute-1. The lighting is poor, but there is a diesel generator available. The child weighs 17kg.
Preoperative Evaluation and Plan
The surgical skill level available in the rural hospital is critical when considering this type of surgery. Is this an experienced surgeon who can adjust to the environment and will be able to retreat and stop surgery if direct visualization of the mass demonstrates a very difficult excision? Can the surgeon operate safely and efficiently (i.e. fast)? You need to consider these types of questions when working in extremely remote regions with challenging surgical and anaesthesia cases.
The only laboratory test needed in this scenario would be a haemoglobin level and a type and crossmatch for one unit of blood. If payment for treatment is required, do not spend the family’s limited funds on laboratory tests that will not change your anaesthetic management. In even the very remotest of locations, there should be a blood bank for family donors and a pharmacy near the hospital that sells the blood and IV giving sets for the family to purchase and bring to the operating theatre. A laboratory that is able to check for HIV, hepatitis, malaria and blood typing would be beneficial. Always remember in an emergency situation a full cross match does not need to be done; give type-specific blood if the patient’s vital signs cannot wait for the full cross match. This specific case would prompt the purchase of two blood giving sets so that if bleeding occurs and one filter blocks, you would have a secondary giving set ready. induction and maintenance of anaesthesia.
A suitable anaesthesia plan in this situation would be total intravenous anaesthesia (TIVA) (combination of ketamine and propofol), and endotracheal intubation. A peripheral IV catheter is placed, 18 or 20 gauge, and intravenous induction performed with succinylcholine and thiopental, propofol or ketamine. Succinylcholine has a short duration of action, which will allow for spontaneous respiration to return quickly and avoid the need for positive pressure ventilation. This allows for a greater margin of safety in case the generator powered oxygen concentrator malfunctions and you are forced to use a self- inflating “Ambu” bag with room air only. Any size “Ambu” bag will be suitable, but take care to avoid excessive tidal volumes if you use an adult “Ambu” bag for a small child. Monitor the chest expansion carefully and do not squeeze the bag totally: note that some “Ambu” bags can give up to 2 litres of volume.
The propofol and ketamine mixture for this rural anaesthesia TIVA technique (“Ketofol”) can be made by adding 20ml propofol 10mg.ml-1 (200mg) and 2ml ketamine 100mg.ml-1 (200mg) to the burette of a paediatric buretrol (microdrop) intravenous giving set. The concentration of propofol (10 mg.ml-1) and the ketamine (100 mg.ml-1) allows one to combine 20mls of propofol and 2mls of ketamine in an approximate 1:1 mg:mg combination for infusion, which simplifies the dosing. Each ml will have 10mg of propofol and 10 mg of ketamine. Most paediatric buretrols have 60 drops of fluid being equivalent to 1ml of fluid which translates to the infusion rates in the table. Confirm the dropper calibration with your specific buretrol being used.
At times, you may need a small dose of muscle relaxant (succinylcholine) but most surgeons can operate with a spontaneously ventilating patient. The goal in the remote environment is to maintain spontaneous breathing, which will add a level of safety. The TIVA technique could be combined with a spinal anaesthetic if the anticipated blood loss is minimal. This would allow for a lower dose of the ketofol, which would be less expensive.
Decrease the infusion rate 15-20 minutes before the projected completion of surgery and stop completely 5 minutes before the end. The patient should be extubated awake, and should be monitored closely postoperatively in the bed closest to the nursing station for 48 hour postoperatively, ideally in a bed that can elevate the head to 30 degrees. Intestinal perforation is a potential risk for this procedure and should be considered if there is any concern about sepsis postoperatively.
| Phase of Anaesthesia | Dose in ml | Dose in buretrol drops assuming 60 drops = 1ml |
|---|---|---|
| Mean induction dose | 0.2 ml.kg-1 | 12 buretrol drops.kg-1 |
| Maintenance infusion: deep sedation (e.g. orthopaedic procedures) | 0.2 ml.kg-1.hour-1 | 12 buretrol drops.kg.hour-1 |
| Maintenance infusion: anaesthesia for laparotomy | 0.3-0.4 ml.kg-1.hour-1 | 18-24 buretrol drops.kg.hour-1 |
| Incremental increases in infusion rate if the patient demonstrates signs of pain [respiratory rate, heart rate, movement] | 0.1 ml.kg.hour-1 extra | 6 buretrol drops.kg.hour-1 extra |
Summary
Children presenting for elective paediatric surgery in sub- Saharan Africa may have a high morbidity and mortality.[3] Understanding the basic anatomy, physiology and pharmacology together with appropriate equipment for the paediatric patient will improve the anaesthesia mortality statistics. The development of paediatric surgical centres in both the rural and urban settings will allow for greater experience to be obtained in paediatric anaesthesia, which will improve care. The most valuable asset for these paediatric centres is to have well-trained physicians and nurses who can provide high quality care for children with the advanced surgical pathology encountered, taking account of the lack of infrastructure and the limited supplies that are a common problem. A successful perioperative course can be expected even for children requiring surgical intervention in austere environments if the basic foundations of anaesthesia are adhered to and if there is a high level of surgical skill available.
References
- ↑ Hadley LG, Rouma BS, Saad-Eldin, Y: Challenge of pediatric oncology in Africa. Seminars in Pediatric Surgery 2012; 21: 136-141
- ↑ Weatherall A, Venclovas R: Experience with a propofol- ketamine mixture for sedation during pediatric orthopedic surgery. Pediatric Anesthesia 2010; 20: 1009-16.
- ↑ Walker IA, Wilson IH: Anaesthesia in developing countries - a risk for patients. Lancet 2008; 371: 968-9.